Before you plan and conduct an investigation, QI, or research project, you must determine if the planned activities to be employed are human subjects research or a quality improvement project (Bass & Maloy, 2020). Specific regulations like the Code of Federal Regulations and Health and Human Services govern the conduct of human subjects research. When the investigator is making this determination, it can be confusing because human subjects research and quality improvement projects share similar characteristics.
While these investigations are both rigorous processes and at times involve similar methods, the two types of investigations have distinctly different overall aims. Quality improvement projects use data-driven methods to improve health delivery and quality through professional practice. Such projects examine changes in human behavior and are largely experiential learning processes associated with the sample and the setting involved. The patient is not an active subject in a quality improvement project but instead the data resulting from their care is examined for any change or improvement. The results cannot be generalized beyond the setting and sample selected in the quality improvement project.
In contrast, research is a systematic investigation designed to develop or contribute to generalizable knowledge which may involve procedures requiring the consent of the patient or participant. The procedures associated with the research are not considered patient care but research measures. Further, visits to the clinic associated with a research project may require consideration as to whether it is even patient care but instead being conducted primarily for research procedures. In this case, should a patient even be charged for the visit if it is research.
Quantitative Methods & Designs
A quantitative method and design is selected when the outcome or dependent variable is numerically measured and analyzed for a statistical difference. The quasi-experimental design is the most commonly used design for quality improvement and research studies as it permits sampling of existing groups which are sampled dependent on the independent variable or quality improvement. This design is also referred to as a nonexperimental, quantitative, quality improvement project.
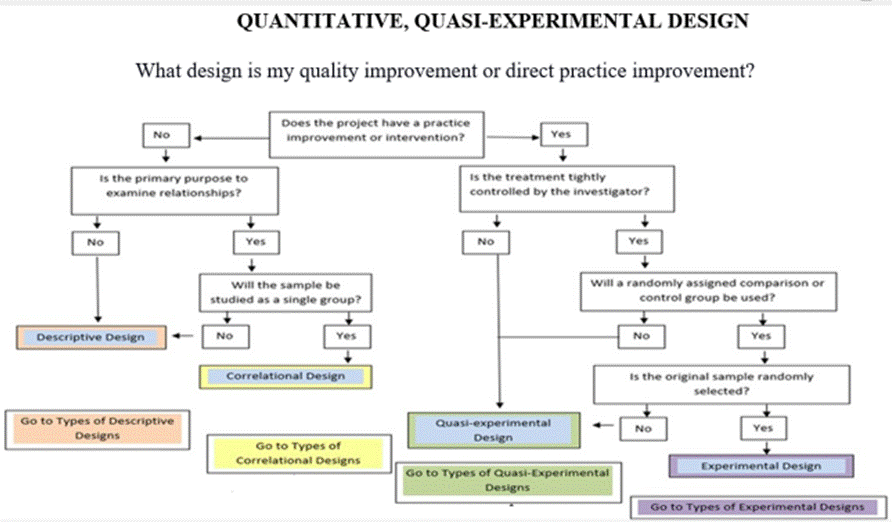
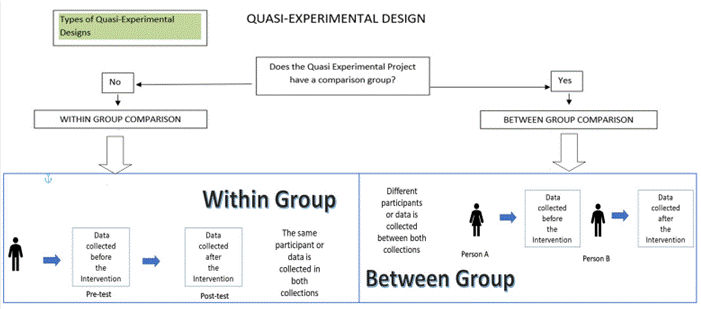
The quasi-experimental design is a quantitative method used to compare groups of data and analyze differences between or among the variables measured. The within group or paired quasi-experimental design compares measurements from the same sample before and after implementing the intervention or quality improvement. The between group quasi-experimental design compares measurements from two non-equivalent groups or samples of data before and after implementing the intervention or quality improvement. Either of these approaches can be used for the quasi-experimental design. Anecdotally, the experimental design uses a combination of both the within and between group comparisons making it the most rigorous design for generalization purposes.
WHAT STATISTICS CAN BE USED FOR ANALYSIS?
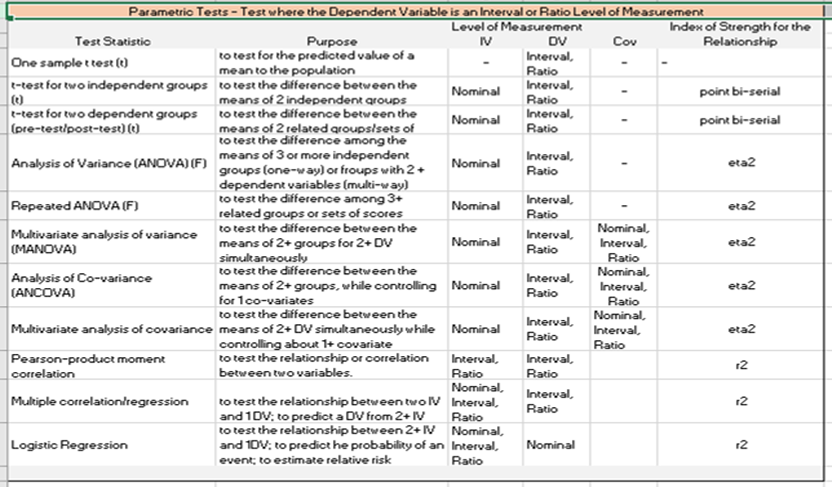
The first decision that must be made when determining the correct statistical analysis is whether the purpose of the data analysis is description or inference. Descriptive statistics summarize data using central tendencies such as mean, median, modes or frequencies to describe the measure or variable. The other data analysis is inferential statistics, which is used to draw conclusions from the results of the data analysis. Inferential statistics are used when specifying a significance level for determining a statistical significance. Examples of statistics used for inferential data analysis are the student’s t‑test, chi-square, and analysis of variance (ANOVA).
Choosing the correct statistical method depends on the following three things: the level of measurement of the data, the distribution of the data, and the nature of the observations (paired/unpaired). There are four levels of measurement to the variables: ratio, interval, ordinal and ratio.
All statistical methods used to compare the means are called parametric, while statistical methods used to
compare other than means (ex‑median/mean ranks/proportions) are called nonparametric methods.
Parametric Statistics for Analysis of Ratio or Interval Level Dependent Variable
Note. Image adapted from Creswell and Creswell (2018).
The independent variable within a quasi-experimental design will be a nominal or categorical level variable identifying the sample or group associated with the intervention. It is the dependent variable’s level of measurement which will direct the type of statistical analysis e.g. parametric versus non-parametric. If the dependent variable is a ratio, interval, the test to be used would be a parametric one. If the dependent variable is an ordinal or nominal level, a non-parametric test would be used.
Non-Parametric Statistics for Analysis of Nominal or Ordinal Level Dependent Variable
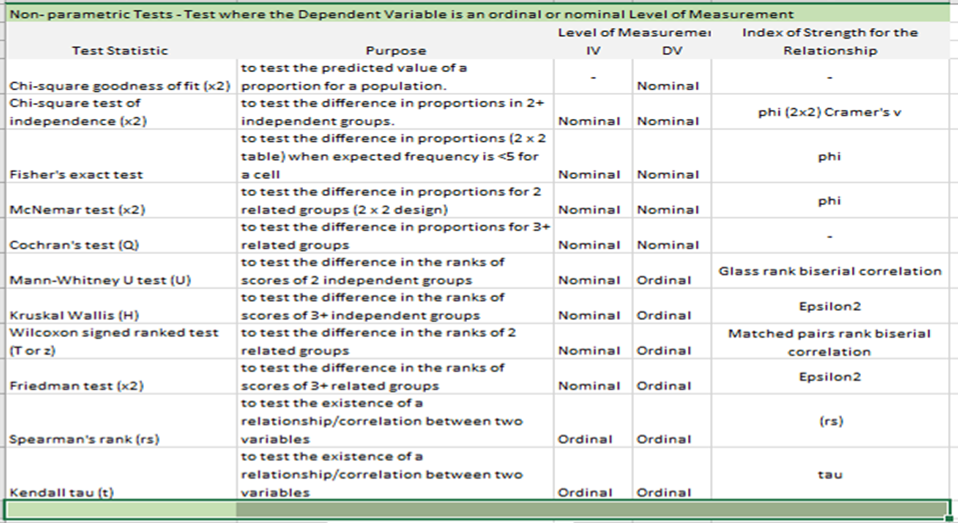
Note. Image adapted from Creswell and Creswell (2018).
American Psychological Association. (2020). Publication manual of the American Psychological Association (7th ed.). Washington, DC. ISBN: ISBN-13: 978-1433832178, ISBN-10: 1433832178
Bass, P. F., & Maloy, J. W. (2020). How to Determine if a Project Is Human Subjects Research, a Quality Improvement Project, or Both. The Ochsner journal, 20(1), 56–61. https://doi.org/10.31486/toj.19.0087
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7122260/pdf/TOJ-19-0087_56Bass.pdf
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic.
Creswell, J. W., & Creswell, J. D. (2018). Research design (5th ed.). SAGE Publications
Melnyk, B. M., & Fineout-Overholt, E., (2018). Evidence-based practice in nursing and healthcare: A guide to best practice. 4th Edition. Lippincott Williams & Wilkins. ISBN-13: 9781496384539
Mishra, P., Pandey, C. M., Singh, U., Keshri, A., & Sabaretnam, M. (2019). Selection of appropriate statistical methods for data analysis. Annals of Cardiac Anaesthesia, 22(3), 297–301. https://doi.org/10.4103/aca.ACA_248_18
IOWA Model
Iowa Model Collaborative. (2017). Iowa model of evidence-based practice: Revisions and validation. Worldviews on Evidence-Based Nursing, 14(3), 175-182. doi:10.1111/wvn.12223
Squire Guidelines
Ogrinc, G., Mooney, S. E., Estrada, C., (2008). The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. BMJ Quality & Safety,17,i13-i3 https://qualitysafety.bmj.com/content/qhc/17/Suppl_1/i13.full.pdf